ABOUT MILITARY SPECIFICATIONS
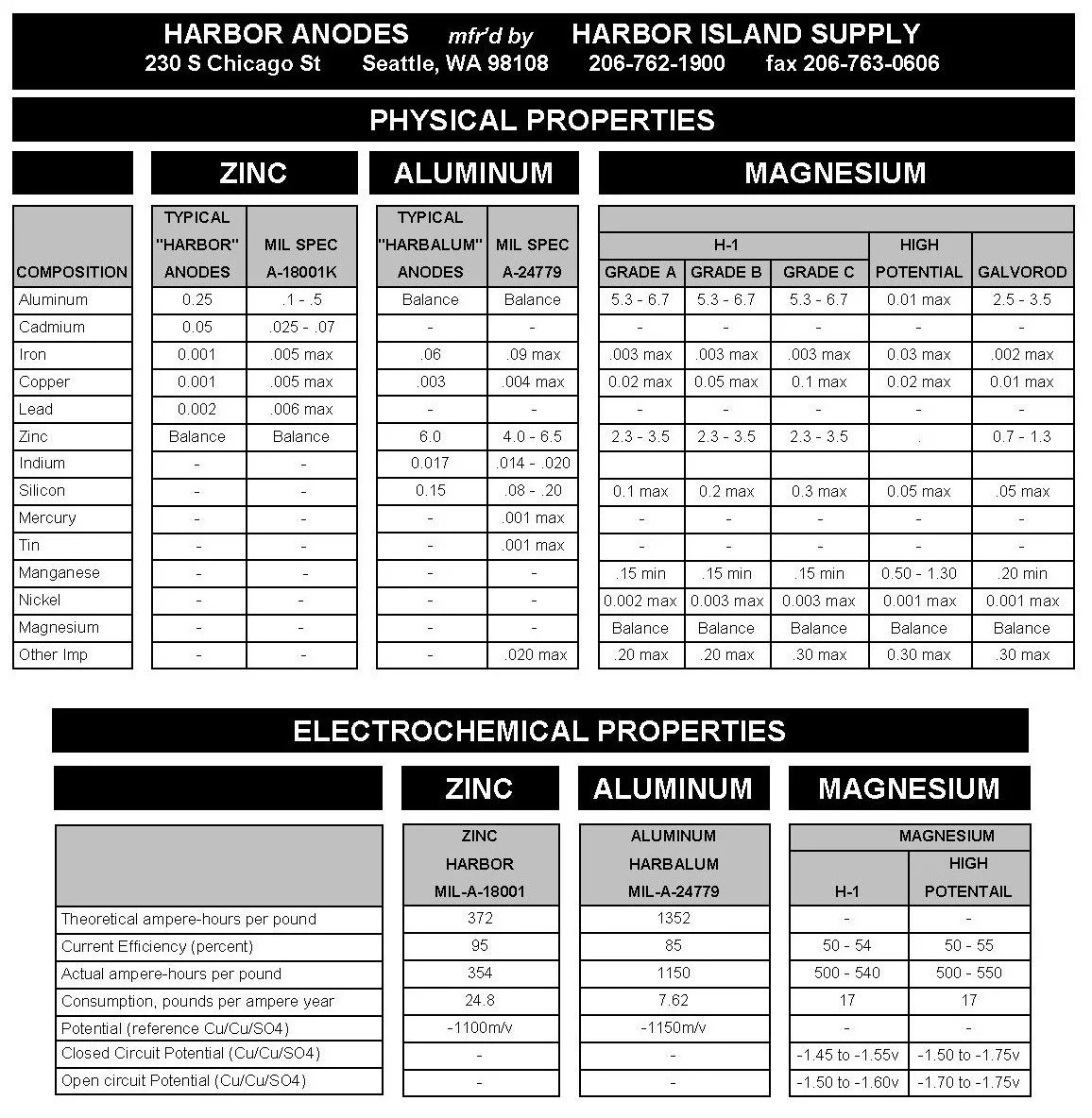
Harbor Island Supply produces zinc, aluminum, and magnesium anodes for cathodic protection.
HARBOR Zinc Anodes conform to the MIL-A-18001K and MIL-DTL-18001L(3) alloy, while HARBALUM Aluminum Anodes meet MIL-A-24779 and MIL-DTL-24779 alloy.
Cast Magnesium Anodes and Packaged Backfill Magnesium Anodes meet AZ63, Alloy H-1 Grade A, Grade B or Grade C. Extruded Magnesium Rod meets AZ31 (GalvoRod Alloy).
New research occasionally upgrades revisions to the U.S. Naval Military Specifications. Our anodes conform to these latest improvements.
-
PROS:
Performs well in salt water and upper levels of brackish waters.
Potential of -1100mv is high enough to protect steel and aluminum.
Sloughs its consumed metal well and retains constant current throughout its life.
Efficiency is high at 95%.
CONS:
Only partially protects in fresh water and lower levels of brackish waters.
Shorter life than aluminum.
-
PROS:
Performs well in salt water and upper levels of brackish waters.
Potential of -1150mv is under the over-voltage point of steel.
Longer life than zinc.
Light weight.
Efficiency is 87%.
CONS:
Maintenance may be required for continued current output, especially in lower levels of brackish and fresh waters.
This alloy doesn’t slough its consumed metal well, which can slow current output and eventually passivate in certain conditions.
-
PROS:
Preferred for freshwater.
The potential of -1450 mv performs well in high resistant fresh water.
Light weight.
CONS:
High voltage in salt water can result in over-voltage (release of hydrogen) from the protected structure, creating excessive corrosion.
More expensive than alternative alloys.
Shortest lifespan.
Efficiency is 55%.
Pros & Cons of Different Alloys
The three main metal alloys used as galvanic anodes are Zinc, Aluminum, and Magnesium.
Each alloy has advantages and disadvantages dependent on many variable situations, including water salinity, water temperature, water speed, current output, passivation, metals to be protected, and the anode’s designed life span. Care should be taken to determine that the best type of anode for given conditions is installed.
Zinc Anodes work well in salt and the upper saline levels of brackish waters. In lower levels of brackish and fresh waters, zinc doesn’t have the current output to fully protect. However, partial protection occurs.
Aluminum Anodes work well in salt and the upper saline levels of brackish waters. In lower levels of brackish and fresh waters, aluminum can film over by not sloughing its consumed metal which causes passivation and the possibility of a total loss of galvanic protection.
Magnesium Anodes work well in salt, brackish and fresh waters. Fresh water use is more prevalent due to the possible “over-protection” that may occur in the less resistant environments of salt and upper saline level of brackish waters.
For vessel protection, typical speed and how often the vessel is used can make a difference in choosing the proper protection. Changing habits in a vessel’s use can also change the type of anode required for protection. Since electrical currents change every time a vessel travels into different environments (waters), there is no absolute value to the corrosion rates of metals. Each vessel or structure must be looked at individually to ensure the proper protection.
Anode 101
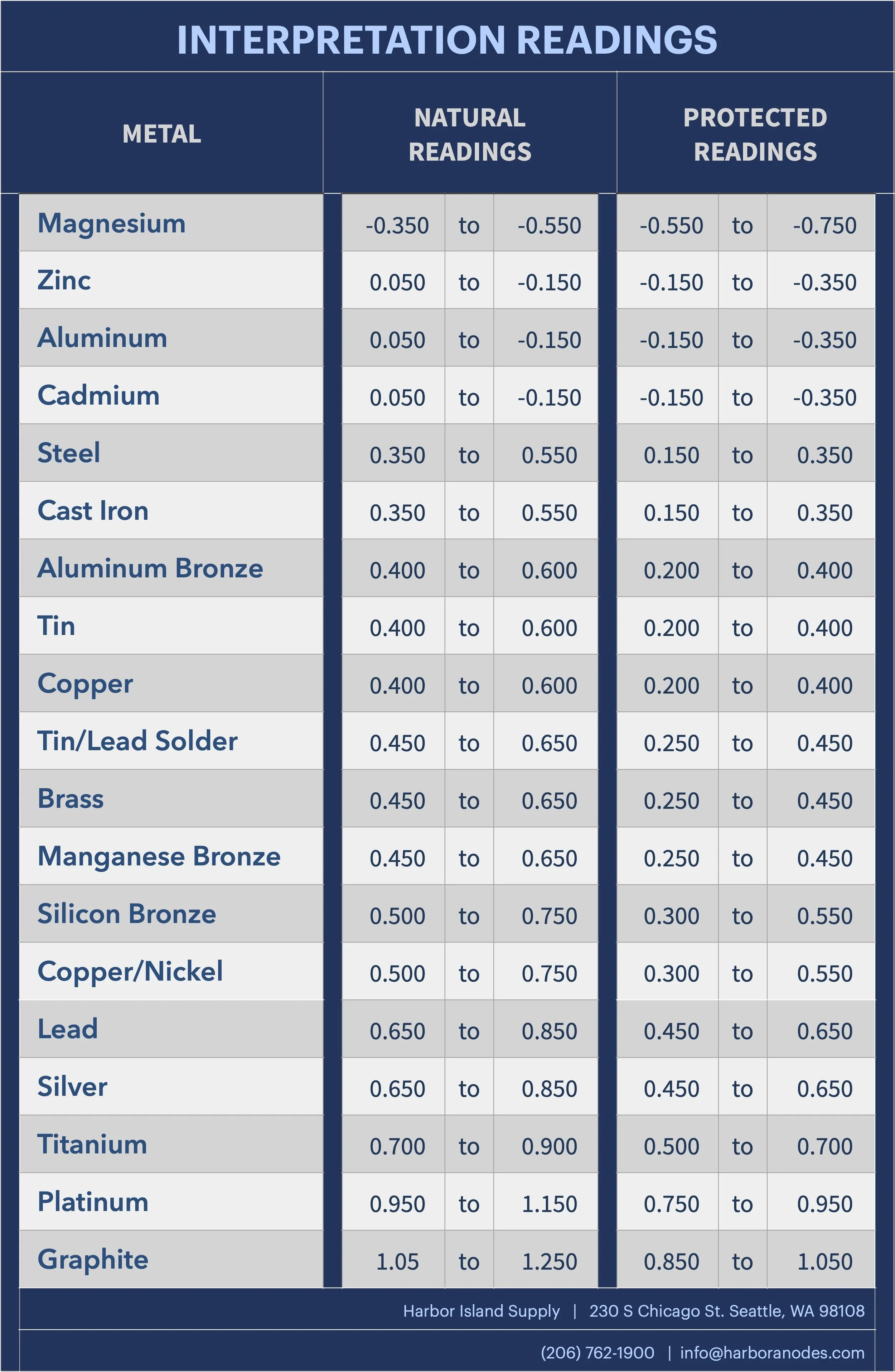
Interpretation Readings
ZINC Reference Cell “Interpretation Readings”
Protected Steel: Cu/Cu/So4 @ -.85v
Protected Steel: Zinc Reference Cell @ -.25v